To apply the steps for creating wave functions we must first establish a potential energy for the electron in an atom. That is: we need to create a model for the atom. We start by noting that the attraction between electrons and the nuclei of atoms is electrical. The potential energy graph for an electron experiencing a one-dimensional electrical force is shown below. This graph looks relatively simple and could be the basis for a model of the atom. However, because the energy is constantly changing, we would have trouble applying the steps to create wave functions. The graph in part (b) simplifies the changing potential. It could be used, but the version in part (c) is even simpler and serves our purposes well. We will use it for our potential energy of an electron in an atom.
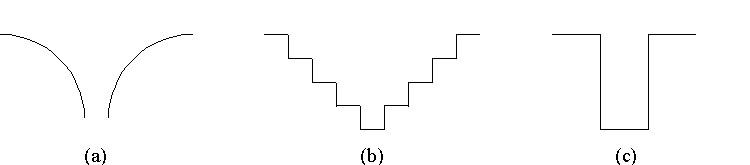
Three possibilities for a potential energy
diagram of a one-dimensional atom.