- Energy Levels in Fluorescence
-
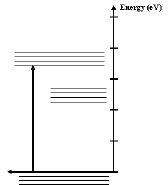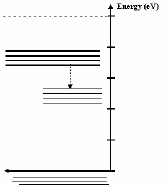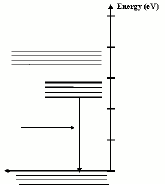
- The first step in any light emitting process is to give electrons
in the lowest energy states enough energy to change to a higher
energy level (Figure 3). In a solid with an impurity band the
electrons in the conduction band do not necessarily lose all
their energy at once and return directly to the ground state.
Instead, these electrons lose enough energy to nearby atoms that
they move from the conduction band into the impurity band. This
transition is represented by a dashed arrow which is illustrated
in the animation.
-
- The output spectrum which we see is the result of electrons
losing energy as they move from the impurity state band to the
ground state band.
-
- Ordinary fluorescent tubes operate when an electric current
passes through a tube filled with mercury gas. The excited mercury
atoms emit visible and UV light. The UV light which has an energy
of about 3-5 eV is converted to visible light by the process
described here.
-



