Rami Arieli: "The
Laser Adventure" Chapter 2.6 page 1
2.6 Population Inversion
We saw that in a thermodynamic equilibrium Bolzman
equation shows us that :
N1 > N2 > N3
Thus, the population numbers of higher energy levels are smaller than the
population numbers of lower ones.
This situation is called "Normal
Population" (as described in Figure 2.3a). In a situation
of normal population a photon impinging on the material will be absorbed,
and raise an atom to a higher level.
By putting energy into a system of atoms, we can achieve a situation
of "Population Inversion".
In population inversion, at least one of the higher energy levels has more
atoms than a lower energy level.
An example
is described in Figure 2.3b. In this situation there are more atoms (N3)
in an higher energy level (E3), than the number of atoms (N2)
in a lower energy level (E2).
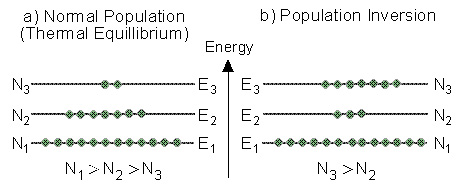
Figure 2.3: "Normal Population" compared to "Population
Inversion".
You can play with an interactive
demo that helps to explain what population inversion
means.
Click on Population Inversion
Demonstration Applet
As we shall see later , this is one
of the necessary conditions for lasing.
The process of raising the number of excited atoms is called "Pumping".
If this process is done by optical excitation (electromagnetic beam),
it is called "Optical Pumping".

