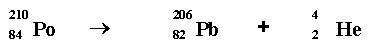
In alpha radioactivity, a nucleus of an atom releases a particle made up of 2 protons and two neutrons. This particle is called an "alpha" particle or a helium nucleus.
Examples of Alpha Radioactivity
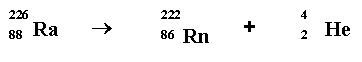
Masses:
Ra: 226.025 amu Rn: 222017 amu He: 4.003 amu
Mass difference = .005 amu
Equivalent to: 4.657 × 106 eV = 4.657 MeV
= 7 x 10-13 Joules.
This common example of radioactivity produces a very small amount
of energy, but much greater than emitted in a chemical reaction.
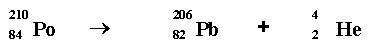
Po: 209.983 amu Pb: 205.974 amu He: 4.003 amu
Mass difference = .006 amu
Equivalent to: 5059 × 106 eV = 5059 MeV =8.9
x 10-13 Joules.
Polonium named by Marie Curie for her native country.
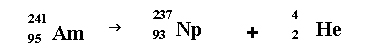
Americium 241 is the stuff in smoke detectors.
Note in all these examples that CHARGE is CONSERVED. The number
of nucleons present is constant i.e. total number of protons +
neutrons does not change. This is true of all types of radioactivity.