 |
|||
 |
|||
 |
|||
 |
|||
Readings
Activity 1A, Potential Energy Diagrams of VQM Activities
Atkins Chapter 27
Homework
Atkins Chapter 27, page 555, Problems 4 and 5
Problems 1 & 2 Activity 1A, Potential Energy Diagrams, pages
1A-9 and 1A-10 of VQM Activities
Problem - Catching a thief Activity 4, Potential Energy Diagrams,
pages 4-3 and 4-5 of VQM Activities
The previous module examined how particles like electrons could behave like waves and concluded with an introduction to the idea of representing matter waves as wave functions. To use wave functions we will need to describe how they interact and this requires an abstract mathematical relationship known as the Schrödinger Equation.
The Schrödinger Equation is commonly written as:
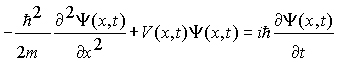
Schrödinger arrived at this equation by incorporating the wave nature of matter, de Broglie's wavelength and conservation of energy. By solving this equation under specific boundary conditions we can determine the wave function and how it changes over space and time. We then interpret the wave function to learn how an object moves and interact over time.
We will be using and manipulating the Schrödinger Equation
graphically rather than mathematically. To do so we will treat
energy slightly differently from what you would have studied in
previous courses. This module is a slight detour in order to look
at energy from a different point of view.