So far we have seen two results of the double slit experiment with electrons
Now we will learn how results 1) and 2) are related to each other and develop a single concept that is consistent with both results - Wave Functions.
Because matter waves are abstract ideas used to describe results,
they do not travel through a medium, such as water. In fact, a
matter wave is not a physical entity at all. So, scientists generally
describe these waves in terms of mathematics or pictures. (We
will use the pictures.)
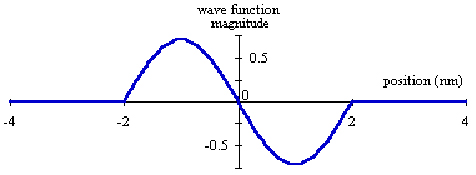
Physicists call the pictures or equations wave functions. A sample
of a wave function of an electron is shown below in figure 2.
The shape of the wave function is NOT the path that the electron
traverses. It is a useful description related to the location
of an electron and can be used to predict the probability of finding
the electron in any given region of space.

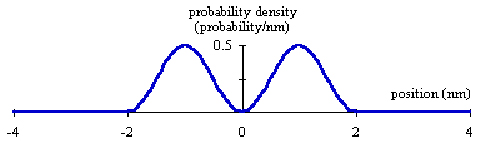
A large amplitude is related to a large probability while a small amplitude goes with a small probability. We have a slight complication. Waves can have amplitudes that are either positive or negative; probabilities must be positive numbers. The solution is to square the wave function. A square number is always positive. The wave function squared appears below in Figure 3.