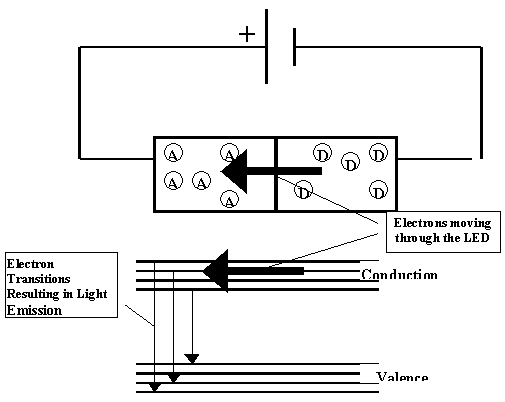
Energy Band Diagram of an LED Emitting Light at the Threshold Voltage
Since the energy bands on the material with acceptor atoms are higher than the bands of the material with donor atoms, the excess, free electrons found in the conduction band of the right block must be supplied additional energy to move to the conduction band of the left block which has a deficiency of electrons. Electrons cannot flow from the left block to the right block because there are not many free electrons on the left block.
When a battery is properly connected across an LED and when the electrical energy (voltage) supplied by the battery is increased, the difference in energy between the right and left energy bands decreases. As the difference in energy decreases, the likelihood of the free electrons moving from the right block to the left block increases. When the appropriate voltage (threshold voltage) is applied, the right and left energy bands reach the same energy level, and the free, energetic electrons of the conduction band of the donor material move to the conduction band of the acceptor material which has fewer electrons. Once there, the energetic free electrons collide with atoms and dislodge their electrons. The dislodged electrons have energies in the conduction band. Within a very short time, however, they are recaptured by their atoms and lose their energy in the form of light emitted by the LED while making the transition to the valence band as shown in the figure below.

The free and excess electrons (which are negatively charged) of the material with donor atoms must be pushed toward the material with acceptor atoms. When a battery is properly connected to an LED, it supplies these excess electrons with sufficient electrical energy to go to the acceptor side of the chip. Because the negative terminal of the battery repels electrons, the donor side must be connected to the negative terminal of the battery so that the excess electrons are pushed to the acceptor side.
The short lead of the LEDs is connected to the side of the
LED chip that contains the semiconductor with the donor atoms.
The long lead connects to the side of the LED chip that contains
the semiconductor with acceptor atoms.
When the positive terminal of the battery is connected to the
donor side atoms, the free electrons are attracted to the positive
terminal of the battery instead of being pushed into the acceptor
side. This situation is shown below. They have insufficient energy
to reach the acceptor side. They can only emit light on the acceptor
side, so no light is emitted.
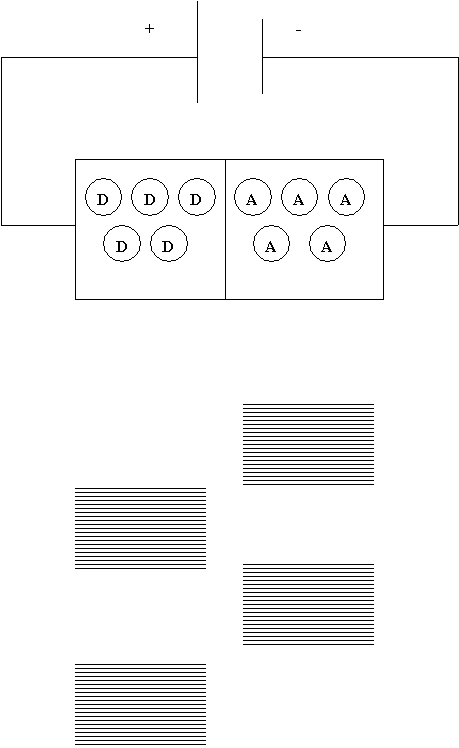
Placing the LED in backwards results in the
available electrons on the donor side having insufficient energy
to move to the acceptor side. No light is emitted.
In our energy level model we have assumed that electrons in a solid acquire and lose energy. Our energy level model of how an LED works, like all models, has limitations. Our energy level model is not perfect and should not be considered a description of all processes that occur in an LED. The model, however, does enable us to explain the general nature of the phenomena that we observed and gives us a general idea of how an LED works at the atomic scale.
We now have gone full circle from exploring the properties of LEDs to constructing an energy level model that explains these properties. The development of this comprehensive model began with atoms of gases to explain the spectral properties of gas lamps and ended with atoms of solids to explain the physical properties of incandescent lamps and LEDs. Along the way we have also learned about gas lamps and the limited number of energies available to an electron in an atom. Knowledge of the energies has been important in almost every area of modern science and technology. One example, the LED and its applications, are important to our daily lives and will play a bigger role as alternate light sources in our future. We could say that the LED and the quantum model that explains how this tiny device works provide us with an "illuminating" experience that allow us to see everyday phenomena in a new "light".